The periodic table
LEARNING GOAL
By the end of this lesson, I will be able to:
SUCCESS CRITERIA
By the end of this lesson, I will be able to:
The Periodic Table of the Elements
In 1869, a Russian chemist named Dmitri Mendeleev came up with a way of organizing the elements that were known at that time. He placed them in order of their atomic weight and then grouped them into rows and columns based on their observed chemical and physical properties. Mendeleev had no idea what atoms were made of or why they behaved as they did, however, he was able to put together the periodic table almost as we know it today. His periodic table had gaps in it since some elements were unknown at the time. Based on the gaps in his table, Mendeleev even succeeded in predicting the existence and properties of several new elements.
The columns on his periodic table are called groups or families, while the rows are called periods.
The periodic table of the elements is a table in which all of the known chemical elements are arranged in order of their number of protons. Elements with similar chemical and physical characteristics are grouped in regions within the table. This is known as the periodic law.
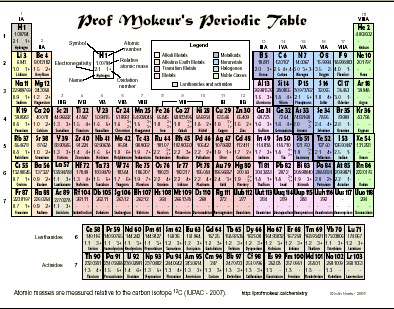 The periodic table can be divided into several families, which are shown on the example above. The main families are the alkali metals, alkaline earth metals, transition metals, lanthanides, actinides, halogens, and the rare gases.
The periodic table can be divided into several families, which are shown on the example above. The main families are the alkali metals, alkaline earth metals, transition metals, lanthanides, actinides, halogens, and the rare gases.
Electron Arrangement
Electrons are found travelling around the nucleus in regions called energy levels. An element's row on the periodic table indicates how many energy levels it can have. Electrons with more energy occupy higher energy levels. Also, there is a maximum number of electrons that can be in each energy level. Elements 1 and 2 can have a maximum of 2 electrons in their energy level, while elements 3 to 20 can have up to 8 electrons in the highest energy level.
Valence Electrons
The electrons located in the outermost, or highest, energy level in an atom are called the valence electrons. Their layout determines the chemical element's behaviour. Also, the layout of the valence electrons from elements in a common group is identical; there are more shells as you go down the group but the outer shell structure is identical.
The Activity Series
Although properties for elements within a column in the periodic table are similar, the intensity of these properties differs. This is called the activity series and lists metals in order of decreasing reactivity.
 The periodic table can be divided into several families, which are shown on the example above. The main families are the alkali metals, alkaline earth metals, transition metals, lanthanides, actinides, halogens, and the rare gases.
The periodic table can be divided into several families, which are shown on the example above. The main families are the alkali metals, alkaline earth metals, transition metals, lanthanides, actinides, halogens, and the rare gases.