Chemical reactions and bonding
LEARNING GOAL
By the end of this lesson, I will be able to:
SUCCESS CRITERIA
By the end of this lesson, I will be able to:
Ionic Bonding
In this activity, chemistry really starts to come alive! In activities 1 and 2 you reviewed the foundations that will be built upon here.
Have you ever wondered how chemistry works? As you learned in Activity 2, a chemical reaction is also called a chemical change. When a chemical reaction occurs, the atoms of the substance rearrange themselves and end up joined to other atoms. This creates a new compound and thus a chemical reaction has happened. But how do they rearrange?
Think back the Bohr-Rutherford diagram that was created in Activity 1. Did you see a pattern in the diagrams? Take a look at the periodic table below, do you see a pattern?
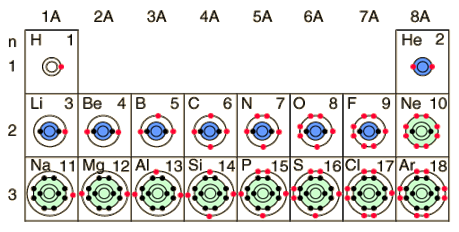 The dots represent electrons. The red dots represent the outermost electrons called valence electrons. Valence electrons are important in determining how the atom reacts chemically with other atoms. The outer most orbital is called the valence shell or valence orbital. Notice in group 1A, the atoms H, Li, and Na all have one red dot. This signifies that they each have one valence electron. In group 2A, the atoms Be and Mg have two red dots, meaning two valence electrons. The pattern continues until the other shell or orbital is full with a maximum of 8 electrons. The first valence shell can hold 2 electrons and then is considered full. For the purposes of this course, the following shells can hold 8 electrons and then they are considered full.
The dots represent electrons. The red dots represent the outermost electrons called valence electrons. Valence electrons are important in determining how the atom reacts chemically with other atoms. The outer most orbital is called the valence shell or valence orbital. Notice in group 1A, the atoms H, Li, and Na all have one red dot. This signifies that they each have one valence electron. In group 2A, the atoms Be and Mg have two red dots, meaning two valence electrons. The pattern continues until the other shell or orbital is full with a maximum of 8 electrons. The first valence shell can hold 2 electrons and then is considered full. For the purposes of this course, the following shells can hold 8 electrons and then they are considered full.
All atoms want to reach a stable electron configuration of eight electrons in their valence shell. The exceptions are H and He who have one shell and are stable when this first shell is full with two electrons. Atoms with a complete shell of valence electrons tend to be chemically inert, which means they will not be a part of a chemical reaction. The atoms Ne and Ar have full valence shells and are unlikely to take part in a chemical reaction. Atoms with one or two valence electrons are highly reactive because the extra electrons are easily removed to form positive ions. Atoms with one or two valence electrons less than a full shell are also highly reactive because of a tendency to gain electrons and form negative ions.
Since the valence electrons determine how the atom reacts and are the electrons that are free to move from element to element, the image below removes the inner electrons and only displays the valence electrons of each element.
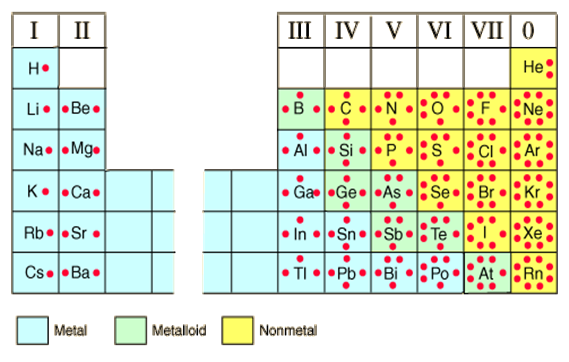 The pattern in the periodic table becomes much clearer. Just showing the chemical symbol and placing dots around it to represent valence electrons is called a Lewis Dot diagram.
The pattern in the periodic table becomes much clearer. Just showing the chemical symbol and placing dots around it to represent valence electrons is called a Lewis Dot diagram.
In group I, which is the first vertical column on the left hand side, the elements H, Li, Na, K, Rb, and Cs all have one valence shell electron. These elements are metals and to become stable they must either gain seven electrons or lose one electron to obtain a full valence shell. It is easier for this group to lose one electron and then the shell under the valence shell becomes the new outer shell and it is full.
On the right hand side of the table is group VII which are called halogens and are mostly non-metals. This group includes F, Cl, Br, I, and At. To obtain a stable electron configuration this group has to gain one electron or lose seven electrons. It is easier for this group to gain an electron to make its valence shell full. Keep this in mind when we start transferring electrons during bonding.
Forming Ionic Compounds
Ionic bonding occurs when a metal completely transfers one or more electrons to a non-metal; the metal needs to lose the electron(s) and the non-metal needs to gain the electron(s) to have stable valence shells.
Ionic bonds:
form between metals and non-metals
are a attraction between a cation and anion
transfer electrons
Example: The reaction between sodium (metal) and chlorine (non-metal):
Sodium (Na) transfers its one valence electron to a chlorine atom that needs an extra valence electron. Na loses loses an electron to become a cation, while chlorine (Cl) gains an election to become an anion. Both atoms now have a stable electron configuration and have bonded to form a stable compound, NaCl. Notice the sodium atom has a hollow dot to represent an electron and chlorine atom has solid black dots to represent electrons. Both symbols mean the same thing; this is done to account for which atoms donate or accept electrons.
Example: The reaction between calcium (metal) and chlorine (non-metal):
Calcium (Ca) has two valence electrons that it needs to lose. Chlorine (Cl) needs to gain one electron therefore, Ca transfers one electron to each of two Cl atoms. Since the Ca lost two electrons it has a charge of +2.
How does Ca have a charge of +2? The atomic number for Ca is 20, which means it has 20 protons and 20 electrons. When you see the diagram for Ca, you recognize it needs to lose two of its electrons to become stable. When it loses those two electrons, it ends up with 20 protons and 18 electrons. Therefore, it has 20 positively charged protons and 18 negatively charged electrons, making its overall charge +2 because it ends up with two more protons than electrons.
In this example, each chlorine atom has gained one electron giving it eight electrons in its valence shell, therefore making it stable. Notice in the empirical formula there is subscript two after the Cl atom; this shows that in order for the reaction between Ca and Cl to occur to make both Ca and Cl stable, one Ca atom and two Cl atoms are required.
Cross-Over Rule
You have learned how metals and non-metals form ionic compounds by drawing their valence electrons and determining the empirical formula from the Lewis dot diagram. Another method of writing formulas that is easier than the electron transfer method is the cross-over rule.
Example: Give the chemical formula and the name for the ionic compound formed between calcium and chlorine.
Step One: Write the chemical symbol for each atom using the periodic table. The metal always goes first.
Ca Cl
Step Two: Write the ionic charge above each of the symbols that you have remembered from the Periodic Table above.
Ca+2 Cl-1
Step Three: Cross-over the ionic charge from the top of each atom to the subscript location of the other atom.
Step Four: Write the ionic charges as subscripts (taking out the positive or negative sign) to the right of each ion to form a chemical formula for the compound. When there is an ionic charge of +1 or -1, the 1 does not need to be written as a subscript.
CaCl2
Step Five: Check to see that the overall chemical formula is neutral.
One calcium atom with a +2 charge and two chlorine atoms with a -1 each produce a zero net charge.
1(+2) + 2(-1) = 0
Therefore, this compound is made up of one atom of Ca and two atoms of Cl. In order to name an ionic compound, you name the first element (calcium) and you name the second element but change the ending to “ide” (chloride).
Calcium chloride
Covalent Bonding and Molecular Compounds
So far, you have learned about ionic compounds; those held together by the attraction of the opposite charges on positive and negative ions of a metal and non-metal. Ionic bonding involves the transfer of electrons from one atom to another.
However, most substances we encounter every day are not ionic. Instead of being composed of a metal and a non-metal, these compounds are made up of at least two non-metals, called a molecule.
In molecules, non-metal atoms share pairs of electrons. Unlike ionic compounds (that are formed by the transferring of electrons to reach a stable outer shell) the atoms of a molecular compound share pairs of electrons to obtain a stable outer shell. These shared pairs of electrons are called covalent bonds.
In terms of covalent bonds, there are three types; single bonds, double bonds, and triple bonds. Note the circles around the chemical symbol and the electrons represent a full valence shell and that the atoms are now stable. Hydrogen has a maximum of two electrons in its valence shell. The short line between the atomic symbols is a short form showing the bond formed by the two electrons. Two lines represent four electrons, three lines represent six electrons. Remember, this is a sharing of electrons to become stable, which is why you see the red circles around the electrons and atomic symbols. Count the electrons in each circle; they all have eight, except for the hydrogen.
Covalent bonds:
form between two or more non-metals
are caused by an attraction between atoms
hold atoms in a molecule together in a certain configuration (molecular structure)
share electrons
 The dots represent electrons. The red dots represent the outermost electrons called valence electrons. Valence electrons are important in determining how the atom reacts chemically with other atoms. The outer most orbital is called the valence shell or valence orbital. Notice in group 1A, the atoms H, Li, and Na all have one red dot. This signifies that they each have one valence electron. In group 2A, the atoms Be and Mg have two red dots, meaning two valence electrons. The pattern continues until the other shell or orbital is full with a maximum of 8 electrons. The first valence shell can hold 2 electrons and then is considered full. For the purposes of this course, the following shells can hold 8 electrons and then they are considered full.
The dots represent electrons. The red dots represent the outermost electrons called valence electrons. Valence electrons are important in determining how the atom reacts chemically with other atoms. The outer most orbital is called the valence shell or valence orbital. Notice in group 1A, the atoms H, Li, and Na all have one red dot. This signifies that they each have one valence electron. In group 2A, the atoms Be and Mg have two red dots, meaning two valence electrons. The pattern continues until the other shell or orbital is full with a maximum of 8 electrons. The first valence shell can hold 2 electrons and then is considered full. For the purposes of this course, the following shells can hold 8 electrons and then they are considered full.
 The pattern in the periodic table becomes much clearer. Just showing the chemical symbol and placing dots around it to represent valence electrons is called a Lewis Dot diagram.
The pattern in the periodic table becomes much clearer. Just showing the chemical symbol and placing dots around it to represent valence electrons is called a Lewis Dot diagram.